Now I’m Positive for Breast Cancer – What’s Next?
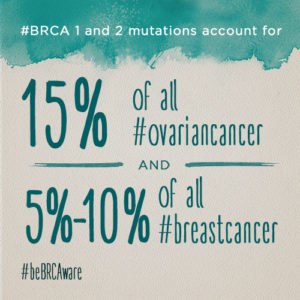
July 27, 2015 (Monday): You may remember that after my Cancer surgery, I had a followup appointment with my Oncologist-OB/GYN surgeon, Dr. Mark Turner. The appointment was mainly to see how I was feeling, take a look at how I was healing and talk to me about having BRCA blood test done. He explained that the research lab would actually test my DNA for mutation which in turn would tell me if I was positive for Breast Cancer.
Maybe most of you know if any of your family members have had breast cancer. In Indonesia it is a little different, people are scared of the hospitals because Indonesians believe if you go to the hospital you never return alive….. I know it is a myth but they actually believe this. I did, growing up but the real reasons for this belief is we don’t have very good hospitals and when Indonesians do go to the hospital it is usually to late to be cured. For most, they just don’t go and and eventually die without the treatment that could have cured them. As example, women don’t get mammograms, pap smears, ect… none of that is available unless you live in the big city and you pay for it yourself. So for me, I would never know what has caused deaths in my family.
Not knowing is kinda scary to me but I was already determined to fight my cancer, I wanted to fight. Knowing if I was positive before I actually had breast cancer would allow the doctors and I to address the issue ahead of time which was important to me. So they drew my blood and that was that.
MY RESULTS: Positive for BRCA 2 – 85% chance of Breast Cancer in my Lifetime
After consulting with my doctors about the results, it seems that since I am receiveing chemotherapy currently which addresses any cancer in my body, that we would address this BRCA issues once we finish the chemo treatments.
MY CHEMO-THERAPY: (6 treatments, 1 every four weeks with blood markers and CATscan before each treatment)
NOTE: I highly recommend the BRCA test to all women.
Remember, Knowledge is Armor!
There is new saliva based BRCA testing that you can do for around $99.
(https://www.color.com/product/brca-genetic-test)
I am not sure if blood based BRCA testing (I did) is better than saliva based testing or
if it makes a difference either way.
I did some research on BRCA testing, here is what I found from breastcancer.org
Article: What to Do if Your Genetic Test Results Are Positive
(https://www.breastcancer.org/symptoms/testing/genetic/pos_results)
If you test positive for an abnormal BRCA1, BRCA2, or PALB2 gene and you have never had breast cancer, you now know that you are at much higher-than-average risk of developing it over the course of your lifetime. The average lifetime risk of breast cancer for women is about 12%. For women who have a BRCA1 or BRCA2 abnormality, the risk of developing breast cancer in your lifetime is between about 40% and 85% — about 3 to 7 times greater than that of a woman who does not have the mutation. Your lifetime risk of ovarian cancer is significantly elevated as well: 16% to 44%, versus just under 2% for the general population. Men with BRCA abnormalities are considered to have a higher lifetime risk of male breast cancer, especially if the BRCA2 gene is affected. One study found that men with a BRCA2 mutation have a 7% lifetime risk of developing breast cancer. They are also at increased risk of developing prostate cancer.
Women with an abnormal PALB2 gene have a 33% to 58% lifetime risk of developing breast cancer. Research on the PALB2 gene continues. While the risk for male breast, pancreatic, and ovarian cancers is thought to be increased, the exact degree of increase remains under investigation.
Whether you are a man or a woman, an abnormal BRCA1, BRCA2, or PALB2 genetic test result means there is a 50% chance you could have passed that specific mutation on to your children.
While rare, it is possible for a person to have one BRCA1 and one BRCA2 mutation. Usually, this occurs in someone with Ashkenazi Jewish ancestry, due to the higher carrier frequency. For someone testing positive for both mutations, screening and risk reduction recommendations are the same as they would be for a person who tests positive for just one of these mutations. In a person who tests positive for both, the risk of passing each mutation to a child is 50%.
Researchers have been working to build their understanding of how breast cancers in women with BRCA mutations may differ from other breast cancers. Some of their findings include:
Breast cancers in women with BRCA1 abnormalities are more likely to be estrogen-receptor-negative — meaning that the cancer’s growth is not fueled by the hormone estrogen — and to have high-grade cell growth. Both of these characteristics mean that chemotherapy will be more effective than hormonal (anti-estrogen) therapy in treating these cancers.
BRCA1- and BRCA2-related cancers often test negative for overexpression of the gene known as HER2/neu. This genetic abnormality is not inherited, as BRCA1 and BRCA2 mutations are, but can develop in women over time. When the HER2 gene is overexpressed, the cancer cells have too many HER2 receptors (human epidermal growth factor receptor). HER2 receptors receive signals that stimulate the growth of breast cancer cells. HER2-positive breast cancer is considered to be a more aggressive form of the disease, but it can be treated with Herceptin (chemical name: trastuzumab), a medication that targets HER2 receptors. Most BRCA1- and BRCA2-related cancers cannot be treated with Herceptin because they are HER2-negative.
Women with BRCA1 or BRCA2 gene abnormalities have no greater risk than other women of having multiple cancers in the same breast when their breast cancer is diagnosed.
A 2005 study suggested that women with DCIS (ductal carcinoma in situ) are just as likely to have inherited gene abnormalities as those with invasive breast cancer.
If you have breast cancer:
If you have a breast cancer gene abnormality and you develop breast cancer, your doctor will work with you to determine how your BRCA status might affect your treatment decisions. For example, if you have a BRCA1 mutation, the breast cancer is less likely to be estrogen-receptor-positive, which means that you may not be a candidate for treatment with hormonal therapy. If you have a BRCA2 mutation, however, you are more likely to be a candidate for hormonal therapy. Because research on the PALB2 gene is ongoing, it’s not clear yet if cancers caused by a PALB2 mutation are likely to have specific characteristics.
You’ll also want to talk with your doctor about reducing the risk of a new, second breast cancer or ovarian cancer. Women with breast cancer and a BRCA1 or BRCA2 abnormality have a significantly greater risk of developing a new, second breast cancer, as well as ovarian cancer.
If you want to lower your risk of a future breast cancer or ovarian cancer:
Whether or not you’ve ever had breast cancer, knowing that you have a BRCA mutation means that you are at much greater risk of developing breast and possibly ovarian cancer in the future. The latest research offers these insights about strategies for lowering those risks:
Preventive or “prophylactic” mastectomy, or removal of both breasts, has been found to reduce the risk of breast cancer in high-risk women by about 90%. After a diagnosis of one breast cancer in a woman with a genetic abnormality, the risk of her getting a new breast cancer is approximately 3% every year (for example, 15% over 5 years). Without a BRCA1 or BRCA2 mutation, the risk of developing a new breast cancer after one episode of breast cancer is only 1% per year.
Preventive or prophylactic salpingo-oophorectomy, or removal of both ovaries and fallopian tubes, can reduce breast cancer risk by as much as 50% when it is done before menopause, because it takes away the body’s main source of the hormone estrogen. It also can greatly reduce ovarian cancer risk. The timing of removing the ovaries differs depending on whether a person has a BRCA1 or BRCA2 abnormality. For those with a BRCA1 abnormality, the recommended timing of removing both ovaries and fallopian tubes is between ages 35 and 40. For those with a BRCA2 abnormality, removing ovaries and fallopian tubes can be considered between ages 40 and 45.
Hormonal therapy medicines: Two SERMs (selective estrogen receptor modulators) and two aromatase inhibitors have been shown to reduce the risk of developing hormone-receptor-positive breast cancer in women at high risk.
Tamoxifen has been shown to reduce the risk of first-time hormone-receptor-positive breast cancer in both postmenopausal and premenopausal women at high risk. Certain medicines may interfere with tamoxifen’s protective effects.
Evista (chemical name: raloxifene) has been shown to reduce the risk of first-time hormone-receptor-positive breast cancer in postmenopausal women. Visit the Evista page to learn more.
Aromasin (chemical name: exemestane), an aromatase inhibitor, has been shown to reduce the risk of first-time hormone-receptor-positive breast cancer in postmenopausal women at high risk. Aromasin isn’t approved by the FDA for this use, but doctors may consider it a good alternative to tamoxifen or Evista. In 2013, the American Society of Clinical Oncology (ASCO) released new guidelines on using hormonal therapy medicines to reduce breast cancer risk in high-risk women. These guidelines recommend that doctors talk to high-risk postmenopausal women about using Aromasin to reduce risk. ASCO is a national organization of oncologists and other cancer care providers. ASCO guidelines give doctors recommendations for treatments that are supported by much credible research and experience. Visit the Aromasin page for more information.
Arimidex (chemical name: anastrozole), also an aromatase inhibitor, has been shown to reduce the risk of first-time hormone-receptor-positive breast cancer in postmenopausal women at high risk. Like Aromasin, Arimidex isn’t approved by the FDA for this use, but doctors may consider it a good alternative to tamoxifen, Evista, or Aromasin.
Hormonal therapy medicines do not reduce the risk of hormone-receptor-negative breast cancer.
Researchers believe that hormonal therapy medicines will likely lower breast cancer risk in women with an abnormal PALB2 gene, but no specific studies have been done yet.
If you want to try to increase the odds of early detection:
Another option besides preventive surgery is to undergo more frequent cancer screenings in an effort to catch cancer early, should it ever develop. Although more frequent screenings do not guarantee early detection of cancer, they are generally recommended for women who do not wish to have preventive surgery.
You can work with your doctor to come up with a screening schedule that is right for you. For example, you might take the following steps:
Begin annual breast MRI at age 25 or mammogram if breast MRI is unavailable. Screening may start sooner if a family member has been diagnosed under the age of 30. Between ages 30 and 75, begin annual mammogram and breast MRI. For women over the age of 75, screening should be considered on an individual basis with their physicians. Men should receive yearly clinical breast exam starting at age 35, and they should start performing breast self-exam at age 35. Consider participating in a clinical trial evaluating newer methods of early detection.
Begin annual screening with pelvic exam by a gynecologist by age 25. Between ages 30 and 35, consider ovarian cancer screening with annual pelvic ultrasound with an intravaginal probe and blood tests for a special protein called CA-125. Consider participating in a clinical trial evaluating newer methods of early detection.
Have a clinical breast exam every 6 months, and examine your breasts monthly.
Consider participating in a clinical trial of cancer prevention strategies.
***************
I listened to this song everyday while I was going through the rough and tough days. Hope it empowers you as it did me.
Fighter (Kane Roberts):


